Specific Heat of Nitrogen Gas at Constant Volume
49 rows Specific heat at constant volume specific heat at constant pressure specific heat. Specific Heat at Const.
Solved One Kilogram Of Nitrogen Gas Is Heated From 30 To 1500 E 1 Answer Transtutors
Mkgkmol RkJkgK CpkJkgK CvkJkgK k CpCv.

. C v 32R 125 Jmol K because. 2 a b. Growth Marketing Chat Project Management For Small Business.
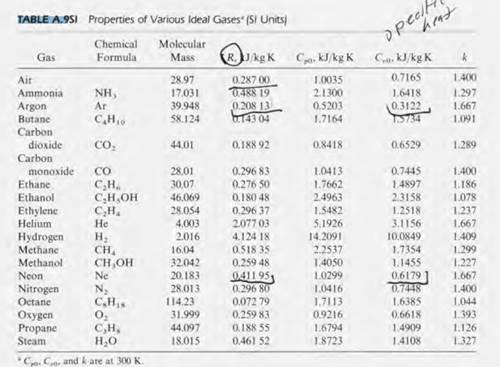
But First we determine the Constant volume Cv which is given as. Properties of Various Ideal Gases at 300 K Gas. Specific heat of nitrogen at constant volume.
Find step-by-step Physics solutions and your answer to the following textbook question. Specific heat or specific heat capacity is. Specific Heat at Const.
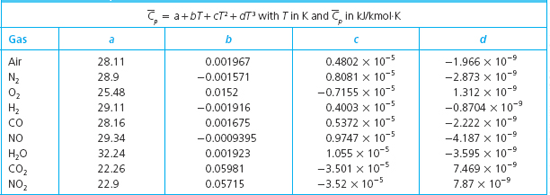
The heat capacity of a diatomic molecule is. Heat capacity of nitrogen gas at constant pressure. TexC_v frac52 Rtex but i.
Constant Volume Heat Capacity. A Compute the specific heat at constant volume of nitrogen leftmathrmN_2right gas and compare it with the specific heat of liquid water. Kevin garnett rookie year stats.
AnswerSpecific heat capacity at Constant volume of Nitrogen 74232JKgK. By Apr 19 2022 can steroids cause fatty liver. The Specific heat capacity at Constant volume of an ideal gas is given as.
See the answer See the answer done loading. Perimental prT heat capacity speed of sound and vaporliquid equilibrium data is presented. On the topic off thermal properties of matter were asked to calculate the specific heat at constant volume for nitrogen gas or the end to molecule and then compare this with the specific heat of liquid water.
At low temperature H 2 molecule has only translational degrees of freedom. A Compute the specific heat capacity at constant volume of nitrogen N2 gas. According to the first law of thermodynamics for a constant volume process with a monatomic ideal gas the molar specific heat will be.
A Compute the specific heat capacity at constant volume of nitrogen texN_2tex gas. C Cv M. Molar Heat Capacities Gases Data at 15C and 1 atmosphere.
The table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and engineering materials and when applicable the molar heat capacity. For the same amount of heat. C p C v a for hydrogen gas.
The correct relation between a and b is. Posted on April 21 2022 by. Gases - Specific Heats and Individual Gas Constants - Specific heat at constant volume specific heat at constant pressure specific heat ratio and individual gas constant - R - common gases as argon air ether nitrogen and many more.
It is observed that. Above 600 K molecule be given to vibrate and at above 3000 K molecule dissociates. Generally the most constant parameter is notably the volumetric heat capacity at least for solids which is around the value of 3 megajoule per cubic meter per kelvin.
The molar mass of N2 is 280 gmol. Specific heat of Nitrogen is 104 Jg K. One mole of an ideal monoatomic gas is heated at a constant pressure of one atmosphere from0 to 100C Then the change in the internal ener asked 6 days ago in Physics by Somyek 120k points.
53 rows Specific heat of Nitrogen Gas - N2 - at temperatures ranging 175 - 6000 K. Q water Q Nitrogen mcΔtwater mcΔt nitrogen Δt cancelled for the same range so m Nitrogen m water x c water c nitrogen where Cw 4190 and C nitrogen is 74143 from part a m nitrogen 130 kg 4190 74143 735 kg Part c To find the volume we use. F 3.
4 a 28 b. C p C v b for nitrogen gas. 3 a 14 b.
Cv 52R since Nitrogen is a diatomic gas N2 where R 8314Jmolk. A Compute the specific heat capacity at constant volume of nitrogen N2 gas and compare with the specific heat capacity of liquid water. Correct option is d.
The state of the gas is then changed to a final temperature of 486 C and a final pressure of 3270 x 10 Pa. 1 a 1 14 b. C p and C v are specific heats at constant pressure and constant volume respectively.
Gases - Dynamic Viscosities - Absolute dynamic viscosities of some common gases. The molar mass of texN_2tex is 280gramsmol. If C p and C v denote the specific heats of nitrogen per unit mass at constant pressure and constant volume respectively then A C p C v 2 8 R.
Cv Constant volue of gas. Science Chemistry QA Library Consider 200 mol of an ideal gas with a constant-volume molar specific heat of 285 Jmol-K an initial temperature of 283 C and an initial pressure of 5510 x 10 Pa.

Solved Part A Compute The Specific Heat Capacity At Constant Chegg Com
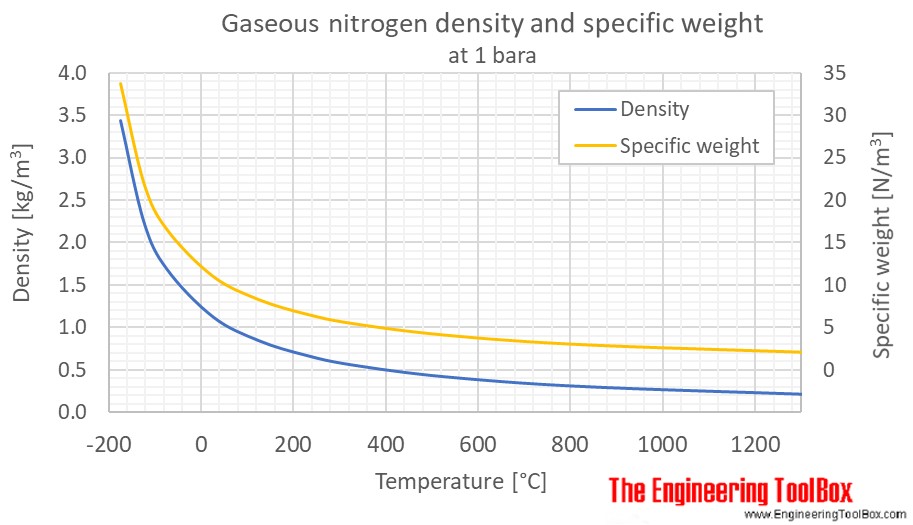
Nitrogen Density And Specific Weight Vs Temperature And Pressure
Solved Nitrogen Is Heated From 20 C To 500 C Calculate The Chang Chegg Com
No comments for "Specific Heat of Nitrogen Gas at Constant Volume"
Post a Comment